How To Make A Ceramic Water Filter
- Inquiry
- Open Access
- Published:
Evaluation of the efficiency of ceramic filters for water treatment in Kambata Tabaro zone, southern Ethiopia
Environmental Systems Enquiry volume 8, Commodity number:1 (2019) Cite this commodity
Abstruse
Groundwork
The Ceramic h2o filtration has been greatly improving the most waste materials from drinking water in developing countries. This enquiry was carried out to determine the efficiency of the Ceramic h2o filters in improving water impurities. The raw materials were grind, sieved with 0.5 mm opening mush size, mixed with water, molded in flower pot shape and fired at 700 °C, 750 °C and 800 °C.
Results
The average removing efficiency of the ceramic filters was found to be 59.half-dozen%, 86.iii%, 87.6%, 56.9%, 59.02%, 88.98%,76.two%, 52.88%, 46.23% and 226.66 m Fifty/h for turbidity, total coliform, E. coli, calcium, magnesium, sulphate, phosphate, iron, nitrite and period charge per unit, respectively in the analysis. Most of the ceramic filters were removed microbial from the contaminated river h2o effectively compared to the Globe Health Organization standard. The results showed that increasing the burn-out textile during production of the filter elements increase the porosity and flow rate while subtract the removal efficiency of turbidity, microorganisms and h2o hardness agent from the source water. Information technology was observed that at that place was a significance difference in removing full coliform, Eastward. coli and flow charge per unit betwixt ceramic filters with different percentage composition of clay to sawdust. The change in percentage limerick of dirt to sawdust and firing temperature did not show significance divergence in removing actions of iron, nitrite, sulphate, phosphate, magnesium and calcium.
Decision
Based on the result, information technology is possible to conclude that the ceramic water filtrate that prepared from 80, 25 and v% clay, sawdust and grog at firing temperature of 750 °C and 800 °C with lower porosity of the ceramic filter elements were institute to be have the all-time removal efficiency.
Introduction
Ceramic h2o filtration has been profoundly improving the about waste matter materials from drinking water in developing countries. In the Globe, one.1 billion peoples exercise not have access to improved drinking-water sources and many people are suffering from diarrhoeal disease (Sobsey et al. 2008). The community faces a tough challenge to have the number people without access to improved water supply. Lack of admission to drinking water and exposure to waterborne diseases from unsafe drinking h2o are issues by many people in the developing countries similar Federal democratic republic of ethiopia (WHO 2012).
Access to safe drinking water is essential to a person'southward for health protection. Recent meta-analysis of field trials has suggested that household-based water quality treatment and condom storage with reducing diarrheal disease (Clasen and Boisson 2006; Clasen et al. 2007). In this context many organizations accept initiate the development of drinking water handling systems suitable for the tropical conditions in developing countries (WHO 2012).
Ceramic silver pot filters (CSF) which fabricated from local bachelor materials are safe to treat drinking water. These filters are low-cost and eliminate approximately 99.88% of h2o born affliction agents for rural signal-of-utilise water treatment (Eneyew and Tesfaye 2017). They tin can be obtained easily and by low cost from local manufacturers. In addition, the filters are effective purifying water made a pot-shape device which removes dangerous microorganisms nowadays in untreated water through the use of gravity filtration.
Ceramic bloom pot too known as the filter elements used a mixture of clay powder and burn down out material are paint with a special solution of silver that kills bacteria. Decontamination can work by pore capture and silver disinfection. When contaminate water is pour the tiny pores inside the ceramic pot act every bit a filter that traps well-nigh particles and debris forth with larger parasites and leaner (Elmore et al. 2005). Silverish nitrate has the result of essentially killing and disabling the reproduction of the smallest leaner that are able to pass through the pores. The silver too helps to prevent mold from growing on the filter surface with time and continued utilise the pores of the filter diminish in size due to clogging and become more efficient in retaining particulates from the water this pot is typically place inside a plastic bucket with a cover lid to help prevent contamination (Howe et al. 2006).
There is different household h2o treatment techniques used in the globe such as boiling, chlorination and bio-sand filtration. All these h2o handling techniques are used to remove the waterborne disease causing microorganisms and to reduce turbidity of the source water. Those techniques are expensive and also reduce the quality of water for example using chlorination as a household h2o treatment reduce or change the gustatory modality of h2o. Chlorine in water combines with natural organic compounds to yield substances such equally trihalo marsh gas. The haloacetic acids and chlorophenols activities to human health (Yang and Shang 2004).
The evaluation of the pre-treatment conditions and fouling behavior of ceramic filter process is prerequisite for the implementation for h2o treatment for drinking h2o production options is adsorption powder activate. Ceramic water filters are reducing the burden of h2o borne illnesses an affordable cost (Huang et al. 2008). However, there is the scarcity of study on the evaluation of ceramic filters capacity to remove water impurities mostly in southern Ethiopia and specially in the study area.
Materials and methods
Description of the written report surface area
This study was conducted in Kambata Tambaro zone, in Hadaro and TuntoWoredas, iii selected kebeles, namely Ajora, Tunto and Lalo. Kambata Tabaro zone that constitute approximately 247 km s-west of Addis Ababa, capital city of Ethiopia. The whole Kambata Tambaro zone is plant betwixt 500 and 3500 meters above sea level and its topography is characterized past steep slope at the human foot of Anbericho, Dato and Ketta Mountains. The zone is boarded on the south by Wolaita, on the southwest by Dawro, on the northwest by Hadiya, on the north past Gurage zones and on the east by Halaba special woredas.
Sample collection and preparation
Clay soils were collected from Ajora, Tunto and Lalo sits. The collected dirt soils were packed, labelled and transported to the drying place. The clay soils were combined with equal proportion and dry out in the sun for 7 days with guidance of the local potters. The clay was grounded with wooden mortar and pestle and screened with 0.five mm sieve size. The saw grit was nerveless from local furniture manufacturers. After drying for 7 days it was processed through wooden mortar and pestle like clay. The saw grit was screened with 0.v mm opening sieve. Grog was grounded with mortar, pestle and sieved using 0.5 mm sieve size.
Mixing the raw materials
The burned material (sawdust) and grog were mixed for 40 min in dry condition with different proportion. Water was added uniformly on a dry out mixture of dirt, saw dust and grog and mixed by wedging and rolling for 40 min to get a smooth homogeneous mixture. Finally, it was divided into blocks every bit shown in the Table 1 (Kabagambe 2010). Ceramic filters were designed in shape of a blossom pot, disc, and candle.
Forming filter elements and firing
Clay mixtures were moulded into flower pot shape in a plastic cup. The pots were pressed to the wall of about more than i cm thickness. The press filter materials were dried in open air for three days and on a drying place for x days to remove excess wet which can cause the filter to crack during the firing process (Babafemi and Yinusa 2015).
Firing was used to produce dense materials and components from ceramic powders by applying thermal energy. After the block clay mixtures were moulded into flowerpot shapes in the desire size, the sunday dry out filter pots were fired in the furnace at different temperature. Different batch of printing dry ceramic filters are place in a muffle furnace and sinter for 5 h at 700 °C, 750 °C and 800 °C with ten °C heating and cooling rates for 5 h. Filter code 1, 4 and 7 were fired at 700 °C, filter code 2, five and 8 were fired at 750 °C and filter code 3, 6 and ix were fired at temperature of 800 °C (Table i) (Joong and Kang 2005).
Ceramic filters test for water treatments
Contaminated river h2o samples were collected from Hadaro Sana River by using standard sampling techniques according to Ilkeret al. (2016). Grab samples of the source h2o were collected with cleaned sterile plastic container and filled in large plastic container, and so, transported to laboratory and refrigerated.
Flow rate test
Flow rate was measured with water taken from the same source Sana River. Source water was filled continuously in the filter elements on testing twenty-four hour period and other water quality parameters were tested in the same solar day. The filter elements fabricated in the above procedure were soaked in pure distilled h2o at least for viii h for constructive and constant flow rate determination in measuring the flow charge per unit of filter elements. It was tested by measuring the amount of water that was percolated after one h. The h2o that laissez passer through the filter flowerpots were nerveless in polyethylene plastic cup then the time and discharged water were recorded on the filter log (Martins 2011). The average period rates were monitored throughout production. The filter elements were cleaned immediately after testing with distilled water.
Microbial removal test
Sana river water was contaminated with indicator microorganisms. This water was diluted with sterilize h2o at 110 °C in 1:1000 ratio by taking 1 mL of the river h2o and diluted with 999 mL sterilize water. The mutual indicator microorganisms used were E. coli and total coliform. A membrane filtration technique was used to find and enumerate total coliform and E. coli from both source water and filtered water samples. The membrane in membrane filtration has uniformly sized holes or pores of diameter 0.45 μm. This pore size is slightly smaller than the diameter of typic, meal total coliform or other bacteria of interest. Equally the water sample was fatigued through the filter, pure h2o passed through the pores, but the total coliform, E. coli and anything larger in size than 0.45 μm were caught on the surface or trapped in the pores of the membrane. The filters were tested for the removal efficiency of microbiological indicators (total coliform and E. coli).
Filter newspaper with 0.45 μm pore size was placed on the filter back up base past using sterile tweezers. The whole apparatus was moved in a swirling motility to stir the sample by pouring 100 ml of the diluted river h2o. Filtration was sprinted and the funnel was rinsed with near xxx ml of distilled h2o twice. The filter membrane was removed carefully with sterilized tweezers and the membrane was so transferred to Membrane Lauryl Sulphate Goop media on metal Petri dish for in a rolling motion. The Petri dishes were inverted and placed into incubator set at 32 °C and 40 °C for 16 h for growth of colony of total coliform and E. coli.
The number of coliform forming units (CFU) were counted under magnifying drinking glass and were expressed equally CFU/100 ml. Microbial removal efficiency were calculated in terms of pct removal efficiency past the following formula.
$${\text{\% Removal efficiency }} = \frac{C\, earlier - C \,later on}{C\, before } \times 100$$
(1)
where C influent microbial concentration in the raw water sample (cfu/100 mL) effluent: microbial concentration in the filtered water sample (cfu/100 mL).
Turbidity reduction test
Turbidity of the water in the influent and effluent water samples were tested by using portable turbidity mete (CL52 D NEPHELOMETER). Turbidity removal efficiency of the ceramic filters was evaluated past using the aforementioned river water. Turbidity of the water samples was measured relative to the turbidity of distilled water having turbidity 2NTU. A pocket-size book of 15 ml of the sample was placed in the sample cell bottle. The exterior surface of the bottle was wiped make clean of fingerprints with the provided cleaning textile before placing in the turbidity meter. First, the turbidity of source water was tested for turbidity before being filtration. So the source water was passed through dissimilar filters. The effluent h2o turbidity was tested after passing through the developed filters.
Finally, the turbidity reduction was calculated past the post-obit formula.
$${\text{Turbidity}}\left( {\text{\% }} \right) = \frac{Turbidity\, of\, source\, water - Turbidity\, of\, filtered\, h2o}{Turbidity\, of\, source\, water } \times 100\%$$
(2)
Water hardness agents removal test
Water hardness removal efficiency of the ceramic filter elements were evaluated with complexation titration method by using EDTA and eriochrome blackness T (EBT) equally an indicator. The concentration of calcium and magnesium cations that cause for the hardness of h2o were measured using complexometry titration in the laboratory prepared solution. 0.0227 M magnesium solution was prepared past dissolving weighted iv.6 m MgClii·6HiiO in 500 mL conical flask with distilled h2o and transferred to 1000 mL volumetric flask and filled with distilled water upward to the mark. Calcium chloride solution was prepared from i.ane g of solid CaCl2 dissolving in a beaker of 500 mL with distilled water and then transferred to m mL volumetric flask and filled with distilled h2o upwardly to the mark.
The titrant 0.01 M EDTA solution was prepared past weighing 3.72 g of EDTA sodium salt dried at eighty °C and dissolved in 500 mL beaker and then transferred to 1000 mL volumetric flask and filled upward to marking. EDTA solution was standardised with prepared primary standard solution of CaCOthree. An approximately 0.01 M solution of EDTA was prepared by dissolving 3.7224 1000 EDTA in distilled water and diluted to 1 L and the solution was standardized with standard calcium carbonate solution complexometrically. EBT solution was prepared by dissolving 0.2 one thousand of EBT indicator in 15 mL ammonia solution and 5 mL absolute ethanol. Buffer solution of pH = 10 was prepared by adding weighed 17 g of NHfourCl in 142 mL concentrated ammonia solution (sp. gravity of 0.9) and diluting to 250 mL with distilled water in a conical flask. To set a buffer solution of pH = ten, 70 g of ammonium chloride was dissolved in 570 mL of ammonium hydroxide (sp gr. 0.90) and marked upwards the book to grand mL with distilled water in a volumetric flask (Birendra and Pandey 2014).
The titration was carried out at a pH of 10, in an NHiii −NH4 + buffer, which keeps the EDTA (H4Y) mainly in the half-neutralized course, H2Y2− where it complexes the Grouping IIA ions very well but does non tend to react equally readily with other cations such as Fe3+ that might exist present as impurities in the water.
From the collected filtered water through each of the ceramic filters 20 mL of the sample was pipated in a clean 250 mL conical flask for each trial and ii mL of ammonia buffer solution followed by 1 mL of EBT and so titrated with EDTA solution. The concentration of metal ion was calculated from 1:i mol ratio of EDTA and metallic ion. The concentrations of the cations were calculated in the samples as (ppm) and finally the removal efficiency of the ceramic filters was evaluated for each code of filter elements. The equation for the reactions that occur at cease betoken of the titration is equally follows:
$${\text{H}}_{ ii} {\text{Y}}^{ 2- } \left( {\text{aq}} \correct) \, + {\text{ MgIn}}^{ - } \left( {\text{aq}} \right) \, \to {\text{MgY}}^{ two- } \left( {\text{aq}} \right) \, + {\text{ HIn}}^{ 2- } \left( {\text{aq}} \correct) \, + {\text{ H}}^{ + } \left( {\text{aq}} \correct)$$
(iii)
Wine red heaven bluewhere; H2Y2− is EDTA, HIn2− is an indicator, MgIn− is metal indicator, MgYii− is metal EDTA complex. For the conclusion of Ca2+ small amount (0.1 g) of magnesium chloride was added in the EDTA because the CaIn− is not very stable and the presence of Mg2+ in the solution ensures a sharp end point through the complexation of the EBT indicator with the magnesium ions present.
Iron removal test
The iron removal efficiency of ceramic filters were performed with unmarried axle UV–visible spectrophotometer (XP-1000P, prc, number 2000 to 8004) for h2o sample taken from Sana river. It was tested in Hawassa University Environmental Enginering Laboratory. In the iron determination, stock solution of Mohr‟s common salt [Fe(NH4)ii(SOiv)2·6HtwoO] 1000 ppm was prepared by dissolving 6.97 one thousand of the salt in 500 mL beaker then transferred to 1000 mL volumetric flask and filled to the marker. Standard solutions at different concentrations (100 ppm, 10 ppm, 8 ppm, half dozen ppm, four ppm and ii ppm) were prepared from stock solution for the scale of UV–visible spectrophotometer instrument. The concentration of one,x phenanthroline was three times the concentration of Mohr's common salt because i mol of Mohrs table salt forms orange red color complex [(C12H8Ntwo)3Fe]2+ with 3 mol of 1,10 phenanthroline equally shown the color in Fig. 1.
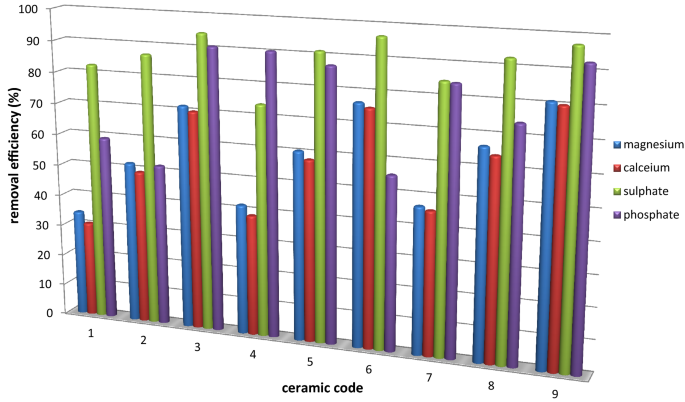
Magnesium, calcium, sulphate and phosphate removal efficiency %
The absorbance of the red circuitous produced was measured with a spectrophotometer at a wavelength of 510 nm. Hydroxylammonium chloride is used as a reducing agent for the conversion of fe (Iii) to iron (Ii) in the solution.
Before UV determination 1 mL HCl, 5 ml hydroxyl ammine, iii ml sodium acetate, v ml ane,x phenanthroline were added by taking 10 ml solution from each of the standard solution and blank solution then the solutions were filled up to mark with distilled water in 100 ml volumetric flask.
The aforementioned process was followed for the source h2o (before filtration) and filtered water (after filtration) past taking 5 mL and 10 mL water respectively then filled upwards to the mark with distilled h2o. Afterward ane h, the UV–visible spectrophotometer instrument was calibrated with standard solution of different concentration and blank solution the absorbance of the source h2o and filtered water was measured and the concentration was calculated with (ppm) based on the equation of the standard solution from the calibration graph at 510 nm.
Nitrite removal exam
The nitrite test was conducted in Analytical chemical science laboratory of Hawassa University using single beam UV visible spectrophotometer (XP-1000P, mainland china, number 2000 to 8004). The laboratory prepared solution of 0.01 G NaNO2 was used to percolate through the ceramic filters and to clarify the removal efficiency of the ceramic filter elements. The stock solution of 1000 ppm was prepared dissolving 1.456 g sodium nitrite in 1000 mL volumetric flask and filled to the marking with distilled water. The standard solutions of (100 ppm, 10 ppm, 8 ppm,half dozen ppm, 4 ppm and ii ppm) were prepared from stock solution for standardization of the instrument. Solution of 0.025 Grand Paranitroaniline and 0.025 M one-naphthol were prepared from the mass of i.726 chiliad paranitroanilin and 1.802 chiliad of 1-naphthol by dissolving in one thousand mL volumetric flask and filled upwardly to the mark separately. Nitrite nether acidic conditions is get through diazotisation with paranitroaniline in ice bathroom and is formed violet colored complex with 1-naphthol under basic condition. All the chemicals used were belittling reagents.
Conductivity and pH test
Conductivity analysis of the source river water and the filtered water through the ceramic elements were performed with Electrical conductivity Meter. The pH of similar source water and filtered water were analyzed with pH-016 pH METER. For conductivity test, standard solution of KCl was prepared with concentration and conductivity of 0.7455, 0.0746 and 0.0149 ppm to confirm the suitability of the instrument. The pH value of 4 and eight buffer solutions were used to sustain the measurement of pH of the source and filtered water.
Porosity of ceramic filters analysis
Porosity of the ceramic filters was determined using the water absorption test (direct) method. Information technology was a subversive method because iii different samples weighing (50 yard, 60 one thousand and 100 k) were taken and the average porosity of these iii samples was the credible porosity of a ceramic filter. The samples were weighed when dry in air then saturated in distilled water at room temperature for xx h. The water with the samples was and so boiled for about two hours and immune to cool to room temperature for some other twenty h. This was washed to ensure that the air in the open up pores of the filter samples was replaced past the distilled water. The soaked samples were weighed under distilled water then removed and surface wiped with tissue paper and weighed in air. The weight of the wire was subtracted from the value obtained while determining the weight of the sample suspended in h2o. Apparent porosity (P) was then calculated using the expression given beneath (D'ujanda 2015).
$$P = 100\left[ {\frac{Wsaturate - Wdry}{Wsaturate - Wunderwater}} \correct]$$
(iv)
where; Wsaturated is the weight of the specimen when saturated in h2o, West dry out is the weight of the dry specimen and Wunderwater is the weight of the sample underwater.
Statistical analysis
Data analyses were performed with the statistical analysis system (SAS Institute 2009). The information generated on efficiency of ceramic filtrates made from unlike proportion of saw dust, grog and dirt were subjected to assay of variance (ANOVA) using the full general linear model procedure. Least meaning (LSD) test was used to decide the differences among the efficiency of ceramic filtrates based on the level at P = 0.05.
Results and discussions
The results obtained throughout the evaluation efficiency process of ceramic filter past varying the ratio of raw materials and firing temperature equally well as the unlike tests done on the contaminates removal efficiency of the filters were discussed in this section. The ceramic filters processed for this piece of work were plant to exist lite gold brown in color (Fig. two). This might due to the beingness of iron(3) oxide in the clay samples (Akpomie et al. 2012).
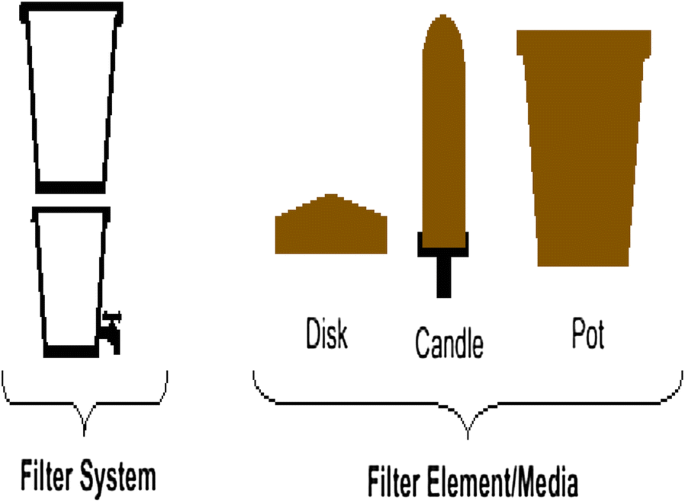
Ceramic filters shape flower pot, disc, and candle
Microbial removal efficiency
The initial concentrations of E. coli and total coli grade in Sana water which was used for microbial removal test were plant to exist 97 and 86 CFU/100 ml, respectively. Every bit indicated in Fig. three, the efficiency of the ceramic filters to remove total coli form and E. coli form bacteria were ranged from 77.32 to 96.51%. As well, the result of this written report showed that the microbial removal efficiency of the ceramic filters increase with increment in the pct limerick of clay and firing temperature. The least removal efficiency registered 77.32% for total coli form and 77.9% E. coli for ceramic water filter code 1. This is nigh probable obtained due to high porosity of the filter chemical element considering of the high pct composition of burn out. Ceramic water filters with relatively low porosity take good efficiency in removing microbial from bacterially contaminated water sources (Clasen et al. 2007). When microorganism pass through the pores of ceramic filters, there might be strong suffocation on their path due to the tourtosity of the path and at the same time they compete for feeding which reduce the number of microbials after filtration (Watters 2010).
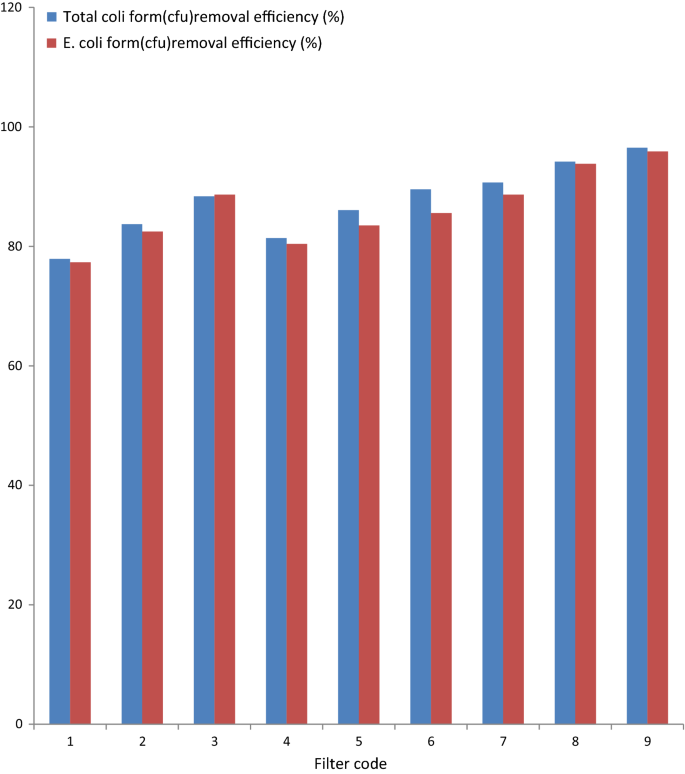
Microbial removal efficiency of the ceramic filters of different code
The upshot of this written report showed that virtually of the developed ceramic filters for this work were found to be constructive in removing microorganisms from contaminated water as compared with WHO guidelines (WHO 2012). Figure three, also shows that the East. coli removal efficiency of all the ceramic filters was relatively higher than the full coli form removal efficiency of the filter elements. The statistical ANOVA analysis confirmed that there was a meaning difference in the total coliform removal efficiency of ceramic filters (p < 0.05).
Ceramic filters with lower percentage composition of burn out code i,2,3 and code 4,v,6 were confirmed no pregnant variation still ceramic filters code 7,8,nine were showed significant variation compared to others filter codes one,2,3 and code 4,5,6. Ceramic filters with similar composition of dirt to saw dust ratio and fired at temperature of 750 °C and 800 °C accept more or less the aforementioned removal efficiency of microbials.
Event of porosity on microbial removal efficiency
Porosity of the ceramic filter is the ground for removing particles in micro size level with physical procedure similar clogging, inertia and adsorption. Figure 4, displays the relationship between Due east. coli removal efficiency (ECRE) and total coliform removal efficiency (TCRE) with porosity of the ceramic filter elements. The result of this study agrees with the finding of Hagan et al. (2008) who reported that less porous ceramic filters are exist able to remove microorganisms efficiently. The fire-outs were leaving smaller pores of about one micron size during ceramic filters firing which can filter out the majority of harmful microbes. Good removal efficiency was observed on the ceramic filter fired at 800 °C with porosity 51.63%. Microorganisms with large size are blocked within pore and retain from flowing through the ceramic chemical element (Doulton 2009). Ceramic filters code 7,8 and 9 had different porosity but they were not differ significantly in removing total coliform and E. colifrom from water (p > 0.05).
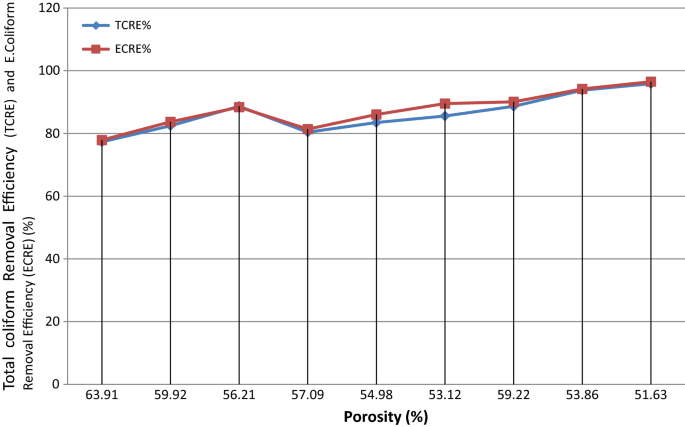
Total coliform removal efficiency (TCRE) and E. coliform Removal efficiency (ECRE%) vs Porosity %
Turbidity removal efficiency
The turbidity removal efficiency of the filters was varied from 31.xi to 82.11% (Table ii). The result of this study confirmed that the turbidity removal efficiency of a filter was affected by porosity of the filter element. As indicated in Table 2, increasing the percentage composition of the burn-out cloth were reduced the turbidity removal efficiency of the ceramic filters. The ceramic pot manufactured with potential to reduce turbidity by thirty% to 100% as reported by Lantagne et al. (2001) and Chung et al. (2013). In many parts of the sub Saharan region the h2o turbidity can be caused by organic waste, silt, bacteria and other germs, and chemical precipitates (Bielefeldt et al. 2009). The statistical assay of turbidity removal efficiency indicates that in that location was no a significance difference in turbidity removal efficiency of the ceramic filters having different percentage composition of clay to saw dust (p > 0.05). Ceramic filters with loftier pct composition of saw dust used in the production of the filter elements code i, 2 and three were greatly differ from ceramic filters code 7,8,9 in turbidity removal efficiency.
Flow rate of the ceramic filters
Equally information technology can exist seen from Fig. 5, the flow charge per unit increases with increase in the flammable cloth that combined with dirt. Such menstruation rate trend of the filter elements might be equally the clay decrease there is a corresponding increment in the combustible materials which burn off leaving networked pores that in plow leads to increment the flow rate of the ceramic pot filters. The flow rates of the ceramic filters were depends on the porosity of the filter elements especially with interconnected pores. The flow rate of code 7 ceramic filter that fired at 700 °C temperature was surprisingly high. This might be the upshot of firing temperature for the formation of relatively high porosity ceramic filters.
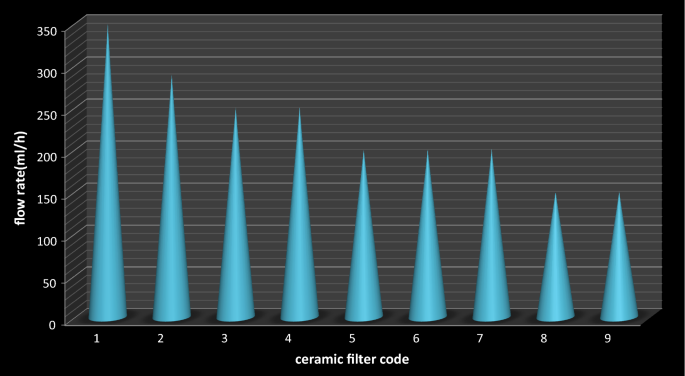
Catamenia rate of ceramic filters
Filters with larger surface surface area have a greater menstruum rate whereas filters with small surface areas take lower flow rates (Halem, 2006). Statistically, there was a significant deviation in the flow rates between ceramic filters with different pct limerick of clay and saw dust (p < 0.05.
Porosity examination
As shown in Fig. half-dozen, the porosity of the ceramic filters was decreased with the subtract in the percent composition of saw grit used for different code of the filter elements. This may be due to filters with more combustible materials have more void space. The porosity of the fired mass is roughly proportional to the book of combustible affair added (Kabagambe 2007).
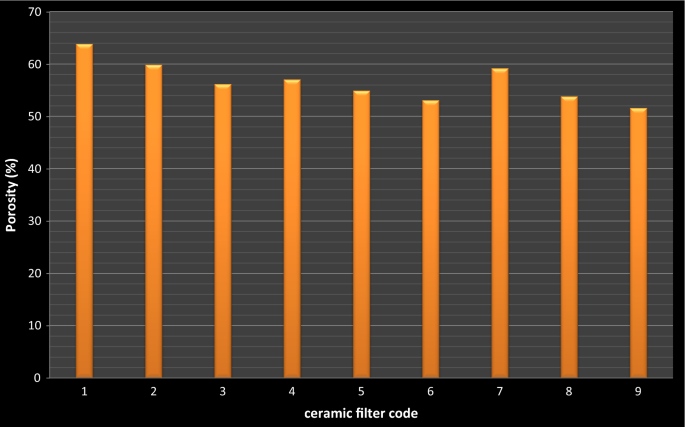
Porosity of different filters with codes
Ceramic filter manufacturing industries take been used upwardly to 200 tone of pressure in molding the filter elements. Such molding materials, could exist exert pressure level homogeneously and remove h2o captivated by green ceramic filter. This is the reason why ceramic filters produced industrially for marketing have relatively lower porosity of average (48%) and free from slap-up while our ceramic filters fabricated in this research work were hand molded and relatively had average (56.66%) higher porosity than commercial ceramic filters that is indicated in (Doris van 2006).
Consequence of porosity on flow rate
Table iii shows that the menstruum rate of the ceramic filters was generally decrease with decrease in the porosity of the ceramic filter elements. The minimum flow rate observed was 150 mL/h for filter lawmaking viii and 9 having porosity 53.06% and 51.63% respectively while college flow rate was 350 mL/h for filter code 1 with porosity 63.91%.
High porosity means there are larger number of void space in the ceramic filters that will allow the h2o to laissez passer through the filter elements. If there are more pores in the filter then water might be flow through faster. The written report results support this suggestion in that as the percentage of sawdust increased for each filter codes so did the flow charge per unit. Porosity in a ceramic water filter allows for water to menstruum through the element (Yoko et al. 2002). The period charge per unit was not only affected by the porosity of the filter element but likewise it was afflicted past the turbidity level of the source water. Source h2o with high turbidity will have low flow rate because the pores of the filters will exist clogged with larger particle sized insoluble solids.
Effect of porosity on turbidity removal efficiency
Figure vii shows that, the turbidity removal efficiency of the filter elements decrease with increase in porosity of the ceramic filters. Ceramic filter with the lowest porosity (49.xiii) was showed higher turbidity removal efficiency 87.93% for the ceramic filters made with similar percentage composition of clay and burn-out but fired at different temperature during the production process. Filters with diminutive pores would exist better at reducing turbidity and microbiological contamination just may have very slow menstruation rates (Yoko et al. 2002).
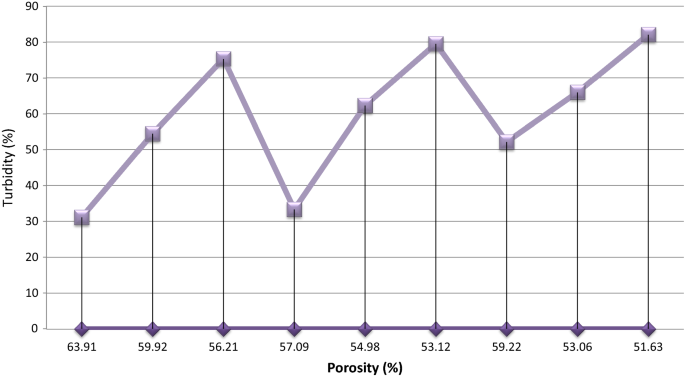
Turbidity % vs Porosity %
Comparing the to the lowest degree significant difference value with the differences between the ways was suggested that removal efficiency of ceramic code iv,five,half-dozen and code seven,8,9 practise not differ significantly from each other. Withal, code i,2,3 were differ significantly from the other groups (P < 0.05).
Effect of firing temperature on porosity
Equally described in Tabular array 4, porosity of the ceramic filters was decreased with increment in firing temperature for the same composition of clay and burn down-out fired at dissimilar temperature. The filters sintered at lower temperature show the highest and the nearly attained porosity as compared to others. This was happened as the sintering temperature increases, the particles attached to like the polymer network movement towards each other and when the network plummet at the elevation temperature can result in shrinkage that tin can effect in reduction of pore size and distribution. As it can be seen from the above Table 4, more efficient ceramic filters from dissimilar percentage limerick of clay to saw dust were attained at 800 °C firing temperature. Moreover, Fig. 8 shows that the event of firing on the porosity of the filter elements in ceramic code one, 2 and 7 indicated that more efficient porosity in removing turbidity and microbial was achieved at 800 °C (code-9). Turbidity and microbial removal efficiency of the ceramic filters fired at 800 °C was more efficient, therefore; firing the ceramic filter at 800 °C (Tables 3 and 5).
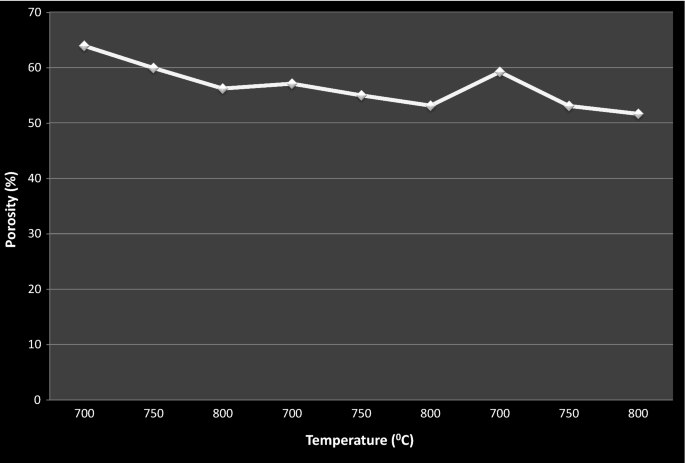
Porosity vs firing temperature
In that location was significant unlike of porosity for ceramic filter fired at different firing temperature, p < 0.05. Statistical ANOVA analysis bespeak that ceramic filters of code one,4,5 fired at 700 °C differ significantly in porosity from ceramic filters having codes two,5,8 and codes iii,vi,9 fired at 750 °C and 800 °C. This analysis indicates that at a higher temperature the clay particles offset to melt and reduce the porosity of the green ceramic by the firing processes. The four stages of sintering are: solid state sintering at this stage the shaped light-green body is heated to a temperature that is slightly different from its melting bespeak and no liquid is present atomic improvidence reduce the porosity, liquid phase sintering a pocket-size amount of liquid is produced and the liquid volume is insufficient to fill the pore infinite, vitrification is a stage where relatively large volume of liquid is formed and sufficient to fill the volume of the remaining pores and viscous sintering is consolidated mass of glass particles is heated near to or in a higher place its softening temperature (Lutgard and Mohamed 2003).
Removal efficiency of water hardness agents
The effect of this study showed that ceramic filters had good removal efficiency of Mg2+ and Ca2+ that crusade for hardness of water (Table 6). The removal of calcium and magnesium ions might be due to the ion exchange on the ceramic surface and formation of atmospheric precipitation as oxides and hydroxides of these cations. The central cations in clay structure, aluminium and silicon with higher charge might exist replaced with lower accuse like magnesium and calcium past leaving net negative charge. More often than not, water hardness removal efficiency of the filter elements increase from ceramic filter code 1 to 9 with decrease in the percent composition of fire-out. The WHO guideline values are given for the concentration of calcium 100–300 mg/50 and magnesium 200 mg/L in drinking water (WHO 2012). At that place was no significance difference in the water hardness amanuensis removal efficiency amidst different code ceramic filters (p > 0.05). A ceramic filter made from saw dust, snails shell glass and clay could be remove 50.8% hardness and 96% fe from steam water by firing with 750 °C temperature (Babafemi and Yinusa 2015).
Fe removal efficiency
At that place is a slight decrease in the removal efficiency with the subtract in percentage composition of the saw dust in the product of filter elements; this might be due to the decrease in porosity of the ceramic filters. Adsorption of cations might be affected by the expanse in contact of adsorbent and adsorbate this surface surface area in contact increase for highly porous ceramic filters.
The interaction betwixt a negatively charged dirt surface and the cations in the pore h2o could be generated an electrical double layer. This was an important phenomenon and this and information technology could be described in terms of the retation of cation in the pore. The primal ions of clay structure might be replaced by others in changing the environment like pH of water in the ceramic pores. From statistical analysis test showed that in that location is no significance difference in iron removal efficiency amidst the ceramic filters formed from different pct composition of dirt and burn-out materials, p > 0.05..
Nitrite removal efficiency
The result of this study showed that the ceramic filters remove nitrite from water solution with removal efficiency minimum eleven.8% of ceramic filter code i and maximum lxx.six% with the ceramic filter code 9 (Tabular array vii). There is a general trend of increase in the removal efficiency with an increase in percentage composition of the dirt with dissimilar codes of the ceramic filters and from the statistical ANOVA test in that location is significant difference between the ceramic filters composed of unlike pct of dirt to saw dust, p > 0.05. The to the lowest degree significant unlike confirms the existence of not bad significant difference in removal efficiency of nitrite between ceramic filters code 1,ii,3 having loftier percent saw dust and filters lawmaking iv,5,half-dozen, lawmaking 7,8,ix with low percentage of saw dust. The nitrite removal efficiency of the ceramic filters might be exclusion within the pores of the ceramic filters according to size.
Resistivity(R), conductivity (cond) and pH of ceramic filters results.
The pH value of the source water was relatively loftier and it was decreased after filtration in all the ceramic filter codes. College pH conditions could heighten adsorption of positively charged ions. There was no significance difference in conductivity of water filtered with ceramic filters made from unlike percentage composition of clay to sawdust, p > 0.05. Electrical conductivity is directly related to the amount of ions present in a given solution (Adhena et al. 2015).
Determination and recommendation
The efficiency of the ceramic filters was evaluated with average removing; turbidity 59.6%, full coliform 86.3%, Due east. coli 87.6%, calcium 56.9%, magnesium 59.02%, sulphate 88.98%, phosphate 76.two%, fe 52.88%, nitrite 46.23% and catamenia rate 226.66 mL/h in the analysis. Near of the ceramic filters were removed microbial from the contaminated river h2o effectively quantity compared to the WHO standard. Effect of different parameters such as per centum composition of clay to sawdust, firing temperature and porosity were investigated for the efficiency of removing microorganisms, turbidity, hardness agents and nitrite of water with the ceramic filter elements. The ANOVA test confirmed the existence of significant difference between ceramic filters in removal of microbials and flow rate which are fabricated from dissimilar percent composition of dirt to saw dust and different firing temperature. At that place is no significant different between ceramic filters made of different percentage limerick of clay to saw grit in removing cations. Using ceramic flowerpot filter for the treatment of water can replace importing household h2o treatment technology and increment the income of local potters. Based on the results observed in the previous part more efficient removal were observed at percent of dirt 80%, sawdust 25% and grog 5% (code-8 and code-9) at firing temperature of 750 °C and 800 °C with lower porosity of the ceramic filter elements were the best removal efficiency.
In order to make the result of this report more practical, the more study need on the social pilot program on the implementation of the ceramic filter in urban and rural expanse of the country.
Abbreviations
- 0C:
-
degree celsius
- WHO:
-
World Wellness Organization
- CSF:
-
ceramic silver pot filters
- EDTA:
-
ethylene diamine tetra acetic acid
- TCRE:
-
total coliform removal efficiency
- ECRE:
-
E. coliform removal efficiency
- CFU:
-
colony forming unite
- ANOVA:
-
analysis of variance
References
-
Adhena A, Belay Z, Angaw K, Jemal Y (2015) Physico-chemical analysis of drinking water qualityatJigjiga Metropolis, Ethiopia. Am J Environ Protect 4(1):29–32
-
Akpomie One thousand, Abuh Thou, Ogbu C, Agulanna A, Ekpe F (2012) Adsorption of Cd(Ii) from solution by nsu-dirt: kinetic and thermodynamic studies. J Emerg TrendsEng App Sce 3(2):253–258
-
Babafemi A, Yinusa D (2015) Formulation of ceramic water filter limerick for the treatment of heavy metals and correction of physiochemical parameters in household water, Ado-Ekiti, Nigeria Scientific Research Publishing Inc. Art Blueprint Rev three:94–100
-
Bielefeldt AR, Kowalski K, Summers RS (2009) Bacterial treatment effectiveness of point-of-use ceramic water filters. Water Res 43:3559–3565
-
Birendra S, Pandey I (2014) Conclusion of calcium and magnesium in clinker, cement and fly, ash based cement by EDTA without using masking reagents. Int J Adv Eng Appl Sci 3(4):9
-
Chung DH, Kimatu JN, Nyariki KO, Su KJ, Onura KN et al (2013) Introducing efficient low cost smoked pots for water purification for developing countries. Hydrol Current Res 4:152. https://doi.org/ten.4172/2157-7587.1000152
-
Clasen T, Boisson S (2006) Household-based ceramic water filters handling of drinking water in disaster response: an cess of a piloprogramme in the Dominican Republic. Water Pract Technol 1(2):1–9
-
Clasen T, Schmidt W, Rabie T, Roberts I, Cairncross Southward (2007) Interventions to amend h2o quality for preventing diarrhoea: systematic review and meta analysis. BMJ 334(7597):782
-
Doris van (2006) Ceramic silver impregnated pot filters for household drinking water treatment in developing countries, Master of Science Thesis in Ceremonious Technology Sanitary Technology Section. Department of Water Management Faculty of Civil Engineering science Delft Academy of Applied science, p 16
-
Doulton (2009) Doulton H2o Filter Ceramic Candle & Cartridge Technologies http://doultonusa.com/HTML%20pages/technology
-
D'ujanda F (2015) Modeling the Porosity Dependence of the Electric Electrical conductivity of Kaolin. Ph.D. thesis, Mamerere University
-
Elmore V et al (2005) Toxicant and parasite challenge of Mainz Intermittent Dull. Environ Toxicol 14(ii):217–225
-
Enyew Amare and Tesfaye Betela (2017) Dirt ceramic filter for h2o handling. Mater Sci Appl Chem 34:69–74
-
Hagan J, Harley Due north, Pointing D, Sampson G, Soam V (2008) Ceramic water filter handbook. Resource Evolution International, Siem Reap
-
Halem D (2006) Ceramic silverish impregnated pot filters for household drinking water treatment in developing countries. Delft University of Technology, Netherlands
-
Ilker Due east, Sulaiman A, Rukayya Southward (2016) Comparison of convenience sampling and purposive sampling Department of Biostatistics, Near East University, Nicosia-TRNC. Cyprus Am J Theor Appl Stat 5(i):i–4
-
Kabagambe M (2007) Performance of ceramic water filters made from selected Uganda clays for bespeak-of-use. Makerere University, Kampala
-
Kabagambe M (2010) Functioning of ceramic water filters made from selected uganda clays for bespeak-of-apply. Makerere University, Kampala
-
Lantagne D, Quick R, Mintz E (2001) Household H2o Handling and Condom Storage Options in Developing Countries: A Review of Current Implementation Practices. Geneva, Water Supply and Sanitation Collaborative Quango
-
Lutgard C, Mohamed North (2003) A paw book of advanced ceramics. Elsevier, New York
-
SAS/STAT 9.1 User's Guide (2009) SAS Institute Inc. U.s.a.; North Carlolina, 2nd edn. SAS Plant Inc., Cary
-
Sobsey SK, Boschi P, Velebit L (2008) Estimating child mortality due diarrhea in developing countries. Bulletin 86(9):710–717
-
Watters T (2010) The effect of compositional and geometrical changes to the angle strength of the ghanaian ceramic pot filter. Masters of Engineering thesis, MIT, Department of Ceremonious and Environmental Engineering, Cambridge, MA, USA
-
WHO (2012) Articulation monitoring program for water supply and sanitation estimates for the use of improved drinking-h2o sources federal democratic republic of ethiopia. WHO, Geneva
-
Yang A, Shang C (2004) Chlorination byproduct formation in the presence of humic acid, model nitrogenous organic compounds, ammonia, and bromide. Environ Sci Technol 38(nineteen):4995
-
Yoko N, Yoshihiro S, Michio I (2002) Sorption Kinetics of heavy oil into porous carbon. Water Res 36:5029
Authors' contributions
AL supervised data collection and interpretation; helped to typhoon the manuscript every bit well as approved the final manuscript. GA collected, analyzed and interpreted the data, which was part of his MSC thesis of Belittling Chemistry at Wolaita sodo University, Ethiopia. Both authors read and approved the final manuscript.
Acknowledgements
We would like to thank, Angecha district, at Kembata Tembaro zone for the scholarship and for fiscal support.
Competing interests
The authors declare that they take no competing interests.
Availability of data and materials
Data generated during this research piece of work can exist bachelor upon request.
Consent to publication
This inquiry work did not involve human subject for experimental purpose and informed consent to participate in the study.
Ethics approval and consent to participate
The upstanding clearance was not required for this item research considering information technology nether included animate being nor plant.
Funding
Financial support obtained from Angecha District, Kembeta Tembaro zone.
Publisher'south Notation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Affiliations
Respective author
Rights and permissions
Open Access This article is distributed nether the terms of the Creative Eatables Attribution 4.0 International License (http://creativecommons.org/licenses/by/iv.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and Permissions
About this article
Cite this article
Bulta, A.L., Micheal, M.A.W. Evaluation of the efficiency of ceramic filters for water treatment in Kambata Tabaro zone, southern Federal democratic republic of ethiopia. Environ Syst Res eight, i (2019). https://doi.org/10.1186/s40068-018-0129-vi
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s40068-018-0129-vi
Keywords
- Ceramic water filters
- Dirt
- Firing
- Micro-organisms and sawdust
How To Make A Ceramic Water Filter,
Source: https://environmentalsystemsresearch.springeropen.com/articles/10.1186/s40068-018-0129-6
Posted by: moradoyoulty.blogspot.com


0 Response to "How To Make A Ceramic Water Filter"
Post a Comment